Could Botox Be a Game Changer for Depression Management?
Dr. Amy Price Neff is a board-certified family physician with a passion for integrative medicine. She practices at MindStream Integrative Medicine in Nashville, Tennessee, combining traditional medicine with tools like mindfulness and Ayurveda to promote overall well-being. Dr. Price Neff has a particular interest in treating depression and is constantly exploring new approaches to help her patients.
In this blog post, Dr. Price Neff dives into the emerging world of Botulinum Toxin (BoTox) as a potential treatment for depression. She explores the science behind the treatment, its effectiveness, and the potential side effects. Ultimately, Dr. Price Neff emphasizes the importance of individualized care and working with a trusted clinician to determine the best course of treatment for depression. Smiley Aesthetics is proud to partner with MindStream Integrative Medicine to offer this promising new approach to managing depression.
This blog post is a great example of Dr. Price Neff’s commitment to patient education and her pioneering spirit in exploring new frontiers in medicine.

Botulinum Toxin (Botox) for Depression??
Is this one of those bright shiny objects of medical therapies? We weren’t sure, but when presented with a treatment that doesn’t involve a daily medication for a common condition that affects many of our patients, we read on! We read recently about botulinum toxin (one brand name is Botox) for depression and decided to get more curious.
Keep in mind that the information in this post is just information and does not constitute medical advice; if you are considering botulinum toxin for any reason, please consult with your treating provider for a thorough discussion of the risks, benefits and intended effects of the medication.
Depression facts:
- Depression is common. From Gallup: “The percentage of U.S. adults who report having been diagnosed with depression at some point in their lifetime has reached 29.0%, nearly 10 percentage points higher than in 2015. The percentage of Americans who currently have or are being treated for depression has also increased, to 17.8%, up about seven points over the same period. Both rates are the highest recorded by Gallup since it began measuring depression using the current form of data collection in 2015.”
- It’s hard to treat. When we use antidepressants such as sertraline, escitalopram, or fluoxetine, we have to treat between 7 to 16 people in order to relieve depression in one person. (This is called a “NNT” or number needed to treat, which is a good way to understand the effectiveness of a treatment or intervention.)
- Depression can be “treatment resistant,” meaning that even when patients seek treatment using medications and other interventions, about 31% remain depressed.
- Not much has changed in the treatment of depression over the last 40 years, which is very frustrating to patients seeking help for these symptoms. But recently, in addition to psychedelics (link to blog) for conditions such as PTSD, anxiety and treatment resistant depression, some new approaches are gaining traction. These approaches include exercise, hyperthermia, and apparently Botox!
What is Botulinum Toxin or BTX?
BTX, which is a serotype A neurotoxin produced by Clostridium bacteria is sold under the brand name Botox Cosmetic®. There are several other serotype A neurotoxins such as Dysport®, Xeomin®, Jeuveau®, Daxxify® and Myobloc®. Botox, while dangerous when accidentally ingested, has been used since the early 1970’s to treat conditions such as muscle spasm, migraine, bladder dysfunction, excessive sweating, and more.
How does it work for depression?
There is no clear answer to this. According to a recent meta-analysis (a type of study that lumps together other studies in ways that help to lower the risk of bias in how we interpret results), the neurotoxin paralyzes the muscles of frowning in the face. This appears to change not only how emotions get expressed, but an individual’s perception of emotion. Changing a person’s physical sensation of how a feeling or emotion is experienced is an aspect of emotional proprioception, that mind-body connection or 2 way street of physical-emotional feedback and experience.
BTX treatments appear to dampen amygdala responses (the part of the brain that responds in fearful, angry, or threatening experiences) to negative stimuli. Strong or even exaggerated responses by the amygdala are common in major depression, post-traumatic stress disorder, anxiety disorders and other conditions. Other hypotheses include social feedback improvements (maybe the individual is putting a more neutral face forward, instead of a depressed frown or scowl.
How is the treatment performed?
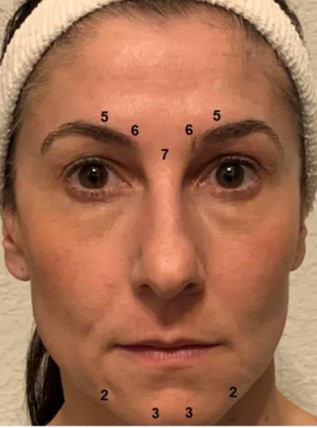
Patients receiving BTX for depression have between 20-50 units of medication injected into the area above the eyebrows, and occasionally along the chin. The eyes, “crow’s feet” are avoided for depression treatment, whereas those are often treated in a cosmetic approach.
How effective is it?
The clinical data for BTX is still fairly small compared to other treatments, but in multiple studies there was an average of a 50% response rate (meaning patients got at least 50% better according to psychiatric rating scales), and a 30% remission rate (meaning the patient no longer met the criteria for the condition for which they were treated). There have been studies that indicate that BTX is at least as effective as sertraline, with some clinical trials showing BTX works more than twice as well as oral antidepressants. There is a strong risk of bias in patients treated with BTX since it’s likely that they are aware of the treatment. Larger trials are to fully establish this approach’s effectiveness.
Is the treatment FDA approved?
Botox cosmetic is currently FDA approved for the glabellar area. At this time, it is not FDA approved for the treatment of depression in this area, but clinical trials are underway. The medication has completed the clinical safety and tolerability studies.
How long does a treatment last?
Clinical trials have followed people for as long as 24 weeks showing the durability of a single treatment, and the antidepressant effect appears to outlast the muscle-relaxing effects of BTX.
Who can benefit?
Most of the studies conducted to date have been in adult women with major depression. There have also been studies with men, as well as studies with in patients with Borderline Personality Disorder, agitated depression, and bipolar depression who have benefitted.
What are some of the concerns about using botulinum toxin as a treatment?
Overall complication rates in BTX treatments are about 16%, with the majority being mild headache or triggering a migraine, or local skin reactions.
Two general types of harm can occur when using BTX.
- Transient or benign side effects, which include pain, swelling, redness, bruising and short-term hypersensitivity of the skin around the location of the injections. These side effects are the same as with any other injectable medication. Botulinum toxin can also cause headaches, allergic reactions, weakness of the muscles that raise the eyelid, and other neuromuscular concerns that while they might not last long, can pose burdensome complications in the short term. Often these relate to paralysis of muscle fibers around the eye that control tear formation, pupil dilation, or eyelid function.
- Serious side effects include events that affect muscles of swallowing, breathing, or severe allergic reaction and are more common in patients getting botulinum toxin for non-cosmetic reasons, thought to be because the dose for non-cosmetic indications is 4 times higher than for cosmetic procedures on average. Botulism is also a risk from BTX. The symptoms of botulism are classified as mild, moderate, and severe, and in the latter categories, cause severe respiratory issues that can require prolonged treatment.
Botox carries a “black box warning” that states: WARNING: Distant Spread of Toxin Effect See full prescribing information for complete boxed warning. The effects of BOTOX and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity, but symptoms can also occur in adults, particularly in those patients who have underlying conditions that would predispose them to these symptoms.
Vigilance about the symptoms is important: breathing difficulty in someone who has had botulinum toxin injection should be taken very seriously, even if the symptoms occur days to weeks later.
Another concern about botulinum toxin in patients that we care for commonly includes immune system reactions. BTX has been safely used in patients with certain autoimmune conditions, such as myasthenia gravis. But in patients with thyroid conditions, there is a possibility that the protein in Botox could aggravate thyroid complications, especially Graves’ Disease and Hashimoto’s. That is a lot of you, my friends!
What does MindStream recommend?
We feel that the most important things to consider when being treated for depression is how effective the treatment is, how acceptable the treatment is in terms of side effects, and whether a person is getting better. Having tools in the toolkit, such as BTX, expands the options for people who are seeking relief from depression. The data presented are promising but not perfect. The treatment, while it appears to be low risk, is not NO RISK. Each person needs to work with their treating clinician to weigh their options.
We work with Smiley Aesthetics to provide this treatment. We trust Smiley as a high-quality aesthetic practice that is committed to continuous quality. If you are interested in pursuing an evaluation for this approach to your mood, we encourage you to call us at 615-541-9933 or email at info@mindstreamintegrative.com.
Additional references:
- Arroll B, Elley CR, Fishman T, Goodyear-Smith FA, Kenealy T, Blashki G, Kerse N, Macgillivray S. Antidepressants versus placebo for depression in primary care. Cochrane Database Syst Rev. 2009 Jul 8;2009(3):CD007954. doi: 10.1002/14651858.CD007954. PMID: 19588448; PMCID: PMC10576545. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10576545/
- Zhdanava M, Pilon D, Ghelerter I, Chow W, Joshi K, Lefebvre P, Sheehan JJ. The Prevalence and National Burden of Treatment-Resistant Depression and Major Depressive Disorder in the United States. J Clin Psychiatry. 2021 Mar 16;82(2):20m13699. doi: 10.4088/JCP.20m13699. PMID: 33989464. https://pubmed.ncbi.nlm.nih.gov/33989464/
- Witmanowski H, Błochowiak K. The whole truth about botulinum toxin – a review. Postepy Dermatol Alergol. 2020 Dec;37(6):853-861. doi: 10.5114/ada.2019.82795. Epub 2019 Feb 5. PMID: 33603602; PMCID: PMC7874868.
- Jara Schulze, Insa Neumann, Michelle Magid, Eric Finzi, Christopher Sinke, M. Axel Wollmer, Tillmann H.C. Krüger. Botulinum toxin for the management of depression: An updated review of the evidence and meta-analysis. Journal of Psychiatric Research, Volume 135, 2021, Pages 332-340, ISSN 0022-3956, https://doi.org/10.1016/j.jpsychires.2021.01.016.
- Gregoric E, Gregoric JA, Guarneri F, Benvenga S. Injections of Clostridium botulinum neurotoxin A may cause thyroid complications in predisposed persons based on molecular mimicry with thyroid autoantigens. Endocrine. 2011 Feb;39(1):41-7. doi: 10.1007/s12020-010-9410-9. PMID: 21061092.